
Then we'll connect the electrons into bonds. Because B needs to make 4 bonds, we'll give it the extra electron. Remember to count +1 for the negative charge on the ion. We count electrons: (3 + 7 x 4 + 1) = 32. In this case, we have to put B in the middle, because F shouldn't make more than 1 bond. (These steps are shown in the picture).įor a second example, let's do the tetrafluoroborate ion, BF 4 –. When I'm putting the electrons in, I usually start by putting each atom's valence electrons around it, then I connect the dots into lines.
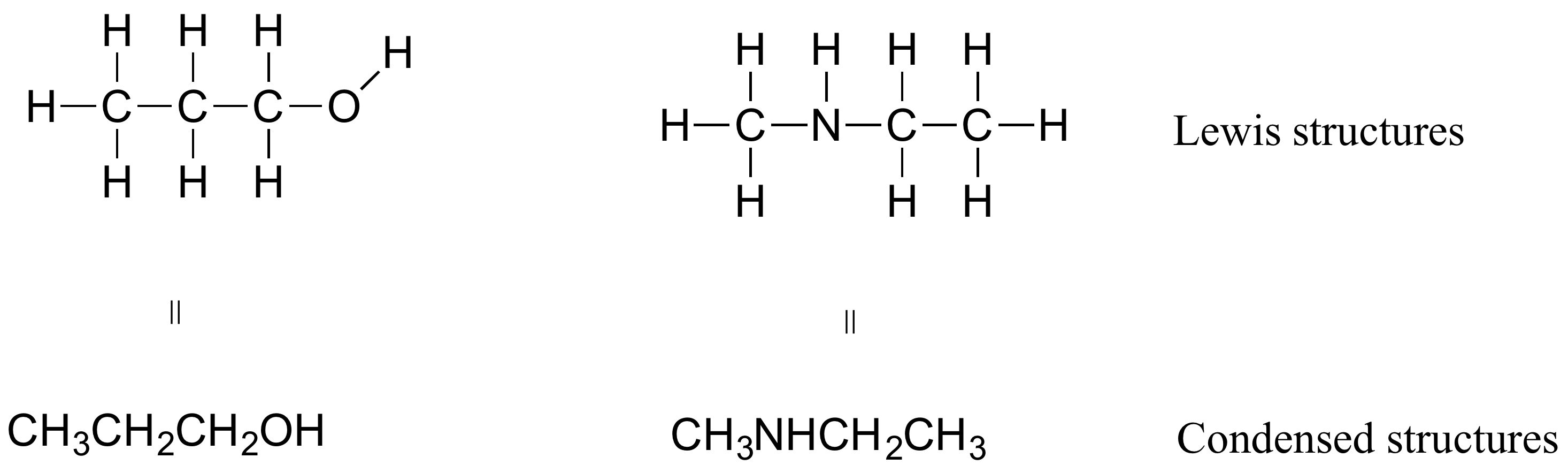
The number of valence electrons in the molecule is (1 + 4 + 5) = 10. As usual, this is the correct order of the atoms. Sometimes connects to 2 atoms, such as in H-bondingįirst, let's do hydrogen cyanide, the poison that might have killed Lewis. Sometimes connects to 3 atoms, such as in H-bonding Sometimes connects to 2 atoms, in H-bonding or with boronĬan be shown as ionic (0 electrons) or covalent (4, 6, 8) Table \(\PageIndex\) : Acceptable Numbers of Electrons and Connected Atoms for Common Elements Element (There are molecules, like O 2, which have unpaired electrons even though they could all be paired, but you can't predict that with Lewis structures, so assume they are all paired.) All electrons should be in lone pairs or bonding pairs. When you draw the Lewis structure, make all the electrons paired unless there is an odd number of electrons.

Unpaired electrons are called radicals, and you should avoid them. Make sure your final structure has the right total number of electrons, and that none of the atoms have too many or too few. Move the electrons around until it works. B often has 6 electrons, and Be often has 4. The heavy elements under C-F should have at least eight electrons, and they can also connect to 6 or even 7 other atoms. Make sure that H has 2 electrons (never more) and C, N, O, F have 8 electrons (never more, and not less unless the molecule has an odd number of electrons). You can draw single, double, or triple bonds. Try to make sure every element gets the right number of electrons, using lone pairs of electrons which are not shared, or shared pairs (which are bonds). Once you have chosen an arrangement of atoms, add the right number of electrons. (Hydrogen bonds, which you may have heard of, are much weaker than the covalent bonds shown by Lewis structures.) H and F will almost always make just one bond. If O connects to 2 atoms, usually at least one is C or H. O should not connect to more than 2 atoms, and often only connects to one. Elements like N, C, S, P, Cl and the heavier elements in these groups can easily connect to 4 other atoms, so often they go in the middle. You should probably not put all the atoms in a line if there are more than 4 (single-bonded chains are usually very unstable, except for carbon). If it is a polyatomic ion, like sulfate or nitrate, usually you put the heavy atom, or the atom to the left in the periodic table, in the center. If the molecule is linear (like HCN) usually it is written in the correct order. You figure out what the connections between atoms are. Count the valence electrons for each atom, add them up, and add or remove electrons if there is an overall charge. Although there are many complicated situations, and some people try to stretch Lewis structures to be an accurate description of molecules even when they don't work well, the basic idea is simple. Describe the interactions between atoms using Lewis structures (what happens to the valence electrons)Įveryone who has studied chemistry should be able to draw Lewis structures. Establish a general procedure for drawing Lewis structures.







 0 kommentar(er)
0 kommentar(er)
